Health Canada Clinical Trial Site Information Form
The Clinical Trial Site Information (CTSI) form is required to be submitted by clinical trial sponsors prior to initiating a protocol or implementing subsequent amendment(s) at the clinical trial site for trials that are subject to Division 5 of Part C of the Food and Drug Regulations or the Clinical Trials for Medical Devices and Drugs Relating to COVID-19 Regulations.
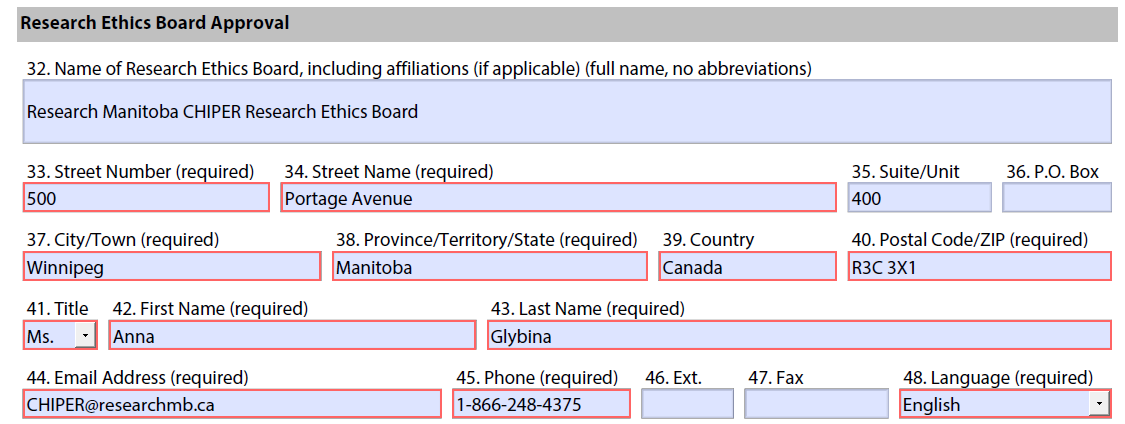
The following information for CHIPER can be entered in the Research Ethics Board Approval section of the CTSI form.
32. Name of Research Ethics Board, including affiliations (if applicable) (full name, no abbreviations): Research Manitoba CHIPER Research Ethics Board
33. Street Number (required): 500
34. Street Name (required): Portage Avenue
35. Suite/Unit: 400
37. City/Town (required): Winnipeg
38. Province/Territory/State (required): Manitoba
39. Country (required): Canada
40. Postal Code/ZIP (required): R3C 3X1
42. First Name (required): Anna
43. Last Name (required): Glybina
44. Email Address (required): CHIPER@researchmb.ca
45. Phone (required): 1-866-248-4375
48. Language (required): English