About Us
RITHIM is a best-in-class provincial program for health research in Manitoba involving multiple stakeholders. RITHIM is unique across Canada in that it harmonizes ethics, privacy and health institutional assessment review processes, creating a more efficient process for health research reviews in Manitoba.
Our Principles
Harmonization
Through frequent consultations with community stakeholders, we support the research community in Manitoba by reducing application complexity through the Harmony online system.
Many complex applications became one.
Privacy
The privacy and security of research data and personal health information is vital to protect.
The Personal Health Information Act (PHIA) amendments of January 1, 2022, brought a NEW REQUIREMENT for researchers. The Provincial Health Research Privacy Committee (PHRPC), administered through RITHIM, is the ONLY committee that has authority to provide approval for research projects requesting USE of personal health information maintained by any Manitoba Trustee, including by government or government agency.
Engagement
Through ongoing engagement with local, national and international partners focused on health research ethics and privacy, we aim to continuously improve and support Manitoba’s research community.
Transparency
RITHIM will provide transparency through the tracking of applications in the new Harmony system and through regular reporting on RITHIM activities and the system. We review our policies and guidelines to ensure that RITHIM remains flexible and adaptable to the changing needs of the research community in Manitoba.
Our History
Concept
RITHIM began development in 2015. It was a simple concept, but a complicated implementation with many variables to consider, requiring a coordinated and collaborative effort for process change throughout post-secondary institutions, the health system and government.
In 2015, researchers identified challenges in the current system for health research approvals in Manitoba. We heard that application processes were numerous, fragmented, difficult to navigate, and that the time to approvals was creating a barrier for research in the province. The provincial government tasked Research Manitoba with forming a Research Improvements Through Harmonization In Manitoba (RITHIM) steering committee to investigate the issue and make recommendations for improvements. A report was generated by the RITHIM Steering Committee and in December of 2017, the government asked Research Manitoba to lead the implementation of the recommendations.
The RITHIM Steering Committee Recommendations
Establish a harmonized review process that encompasses ethics, privacy, and health institutional assessment reviews for health research. One application form.
- Designate an institutional delegate that facilitates the timely review of feasibility of data request and contract process at the approver’s institution.
- Invest in the establishment of an electronic, web accessible, research administration and information system.
- Establish working groups to create bodies of knowledge and act as a forum for discussion around interpretation and application of legislation and the development of provincial working rules and SOPs.
- Provide researcher training and support with navigation.
Implementation
Work began on the implementation of the RITHIM recommendations in 2018 with a focus in the following areas:
| IT Solution – RITHIM Harmony | – Develop a configurable IT system to support RITHIM. This includes establishing the vision for a new system of review, identification of requirements to support the new program and process, creating one harmonized application form, establishing level of services for hosting, rules for user and entity management, migration of active research projects, and comprehensive testing of the system. |
| Operations |
– Establish and staff a new RITHIM operating division. – Support the operations of PHRPC and CHIPER in the transitional state. |
| Change Management and Communications |
-Communicate and prepare for the upcoming change with multiple rounds of engagement with stakeholder groups in Manitoba including post-secondary institutions, government departments, data providers, ethics and privacy review committees, First Nations, Metis and Inuit organizations, health system leadership and Service Delivery Organizations, researchers, etc. This has included workshops, webinars, working groups, communities of practice, presentations and stakeholder meetings. – Develop and implement a Communication and Change Management strategy including the creation of a new website and branding video. |
| Education and Training |
– Provide training opportunities for frontline staff, reviewers, researchers, stakeholders – Develop and deliver robust training for Harmony system users |
Transition Phase: January 1st, 2022 – Harmony Launch (July 2, 2025)
Following proclamation of amendments to the Personal Health Information Act (PHIA), the first phase of RITHIM launched on January 1st, 2022 and operated in a transitional phase until the launch of the Harmony electronic system. During this transitional phase, RITHIM was responsible for the administration and support of the new Provincial Health Research Privacy Committee (PHRPC) and the Committee for Harmonized Health, Impact, Privacy and Ethics Review (CHIPER). Through the transition phase, RITHIM had two important workstreams; operations related to the submission and review of PHRPC applications and CHIPER; and the continuing project work required to complete the software, business process development, and change management necessary to launch Harmony.
Launch of Harmony
The launch of Harmony on July 2, 2025 marked the beginning of RITHIM’s fully harmonized operations. With the Harmony launch, CHIPER embraced its full role as a provincial health research ethics board. Key changes that occurred with the launch of Harmony include:
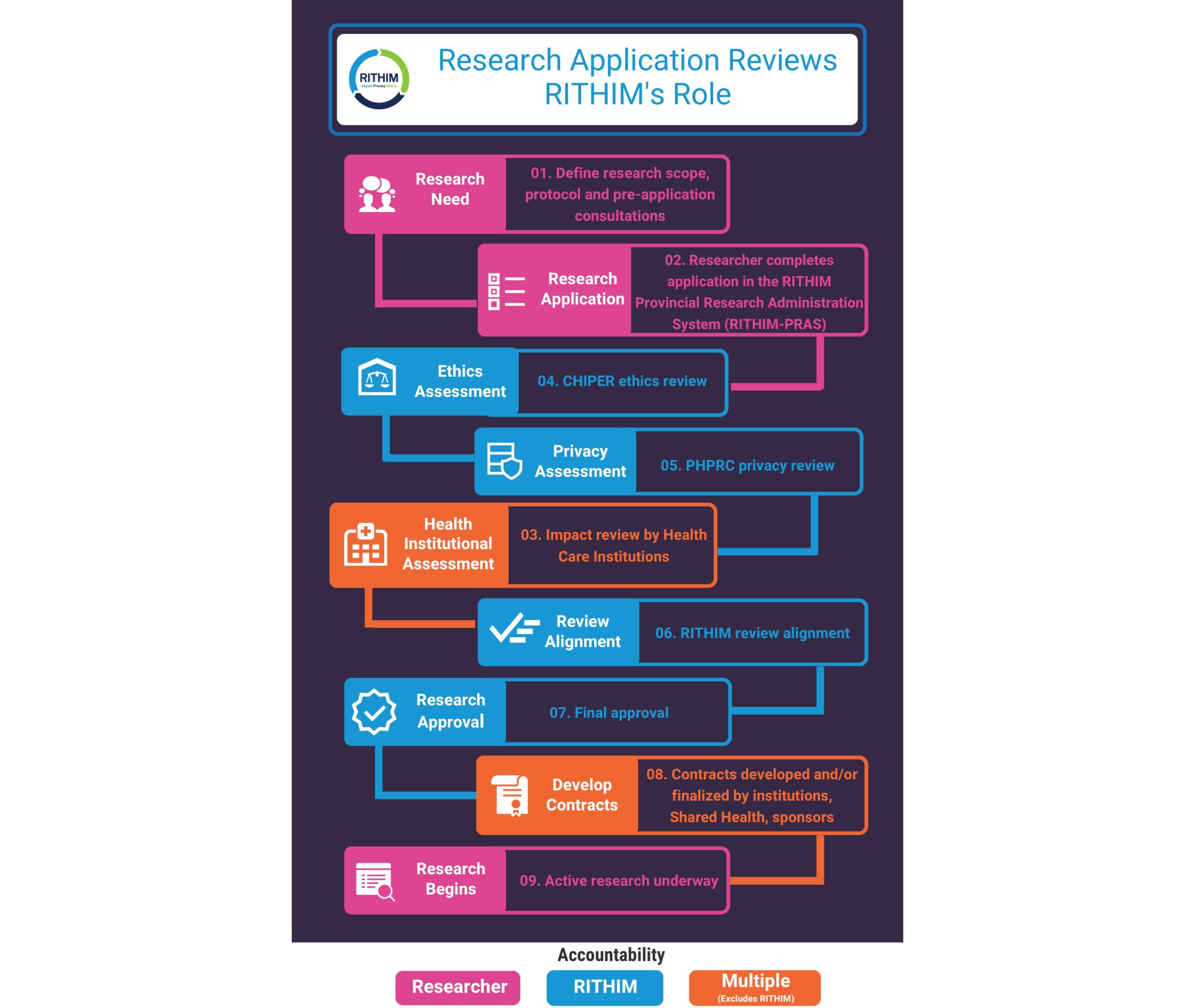
- Researchers conducting health research in Manitoba submit applications for ethics, privacy and health institutional assessment review through the Harmony application form.
- CHIPER conducts health research ethics reviews, supported by the Harmony system and RITHIM staff.
- PHRPC continues to conduct privacy reviews, supported by the Harmony system and RITHIM staff.
- University of Manitoba Bannatyne Health Research Ethics Board (HREB) and Biomedical Research Ethics Board (BREB) stopped accepting new applications.
- Health Institutional delegates receive applications through Harmony, coordinate health institutional reviews of those applications, and provide that input back into Harmony.
- CHIPER, PHRPC and Health Institutional Assessment Reviews are streamlined through Harmony. Researchers receive aligned feedback and decisions from all three perspectives through the system.
Who is Involved?
RITHIM was designed by the research community over a number of years. Key stakeholders in this new provincial program include:
- Provincial Government
- Shared Health, Regional Health Authorities, and Healthcare Institutions
- Post Secondary Institutions and Researchers
- Industry
Stakeholder engagement and collaboration are the foundations of RITHIM. We work closely with our stakeholders and partners is to facilitate engagement, identify barriers and find solutions to common challenges related to the approvals for health research in Manitoba.
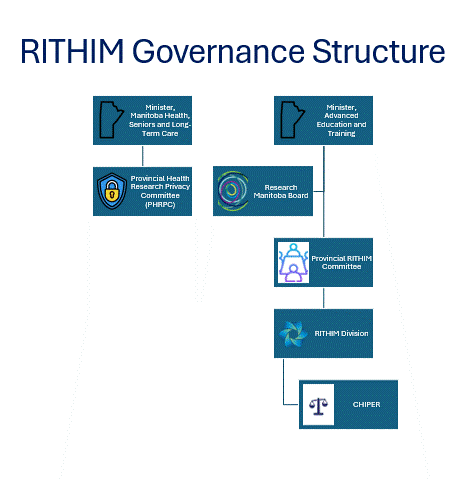
Governance
RITHIM, led by Research Manitoba, coordinates two major committees:
- Provincial Health Research Privacy Committee (PHRPC)
- PHRPC reviews all health research projects that require use of personal health information maintained by any Manitoba Trustee, including government and government agencies, and renders a decision. For more information, please visit the PHRPC page.
- PHRPC members are appointed by the Minister of Health and the committee has been fully operational since January 2022, with administrative support provided by RITHIM staff.
- The full launch of the RITHIM program and the Harmony system supports increased coordination and harmonization with CHIPER ethics reviews and health institutional assessment reviews.
- Committee for Harmonized Health Impact Privacy and Ethics Review (CHIPER)
- CHIPER is a research ethics board (REB) consisting of health professionals, members of the scientific community, and experts in ethics, privacy and access to data. CHIPER’s mandate is to provide robust reviews of health research ethics as outlined in the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans (TCPS2). For more information, please visit the CHIPER page.
- CHIPER was established in January 2022 and operated in a transitional phase up until the launch of Harmony, when it became the provincial REB for health research in Manitoba.
- The launch of Harmony supports increased coordination and harmonization with PHRPC privacy reviews and health institutional assessment reviews.
The Provincial RITHIM Committee (PRC) is a subcommittee of the Research Manitoba board that provides guidance and oversight of RITHIM.
Operations
As of the launch of Harmony, RITHIM is an operating division within Research Manitoba, delivering critical and streamlined services to support the review of health research in Manitoba. RITHIM provides administrative support to CHIPER and PHRPC, and coordinates with health institutional assessment delegates within the health system to streamline reviews. RITHIM processes are supported by an electronic system called Harmony, and the RITHIM team provides training and support for Harmony users. RITHIM has 5 full time staff including a Director, 3 Program Officers and a Support and Training Officer. Additional services to support RITHIM are provided by University of Manitoba staff.
RITHIM Responsibilities Chart