Managing In-Flight and Active Research
Please review the following information carefully for a smooth, informed transition.
Key Terminology to consider:
Active Research – projects that are fully approved and currently enrolling participants or collecting data.
Fully Approved Research – projects where all required approvals have been obtained (e.g. ethics approval, PHRPC if applicable, all applicable health system approvals) but have not yet begun enrolling participants or collecting data. Note: This definition is not dependent on execution of any research agreements or health system site activation.
In-Flight Applications – projects where a portion but not all required approvals have been submitted or obtained.
Migration of Ethics-Approved Projects from the Bannatyne REB
Please be advised of the following important dates with respect to the final import of projects:
- October 29, 2025: last day the Bannatyne office accepted follow-on applications (e.g., amendments, safety updates, major deviations, etc.) for active/approved research.
- November 10, 2025: Harmony system is open for validation of imported projects and subsequent follow-on applications for University of Manitoba applicants.
- November 24, 2025: Deadline for responses to tabled applications to the Bannatyne office.
- December 5, 2025: Deadline for responses to conditional approvals to the Bannatyne office
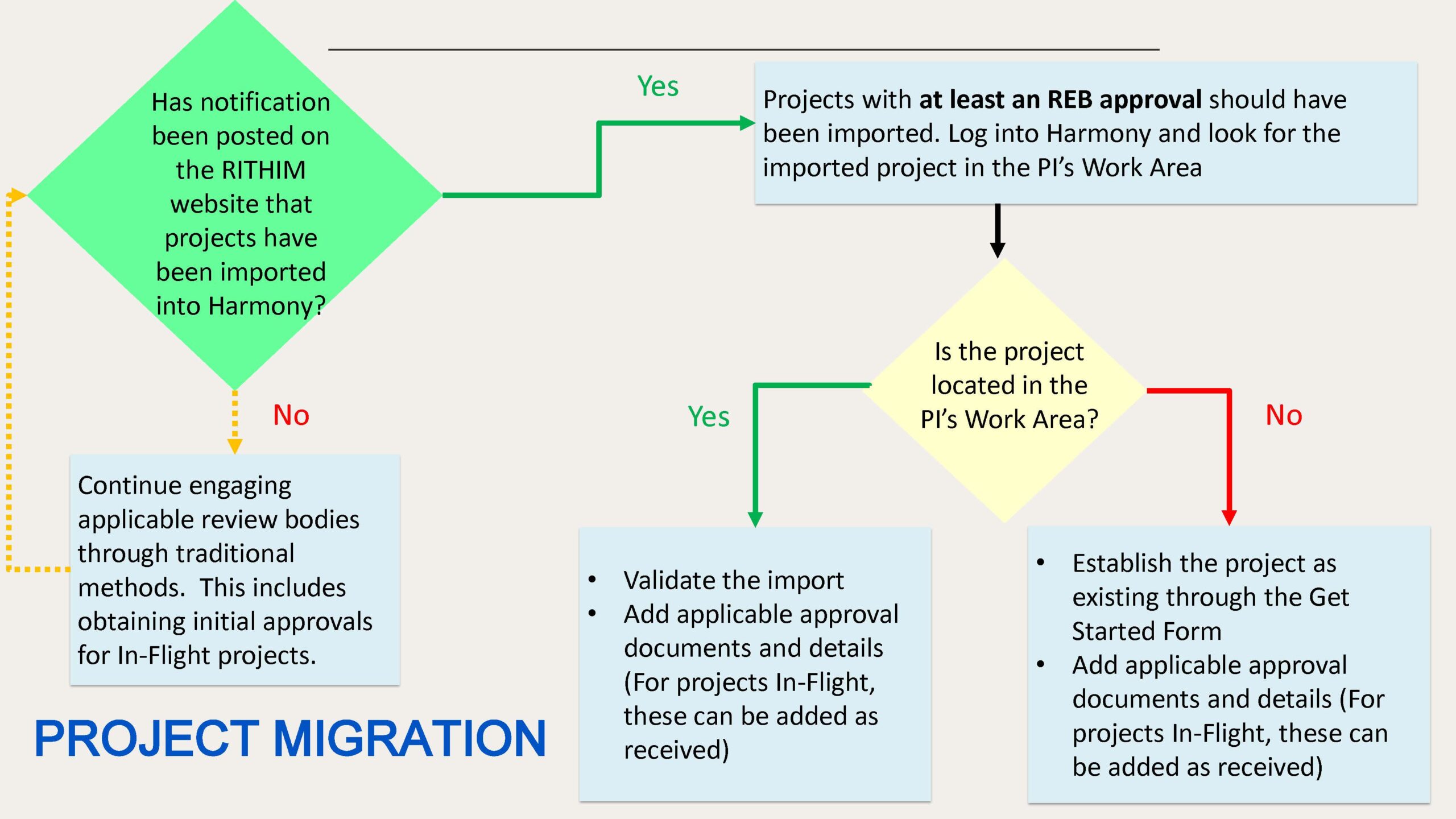
Imported/Migrated Project Next Steps
Once visible in your Harmony Work Area, your imported projects must be validated prior to further processing in the system. Imported projects are validated by reviewing imported information, correcting any inaccuracies, and uploading additional legacy information, applications and approvals.
If your existing project has not been successfully imported or becomes ethically approved after import, you must manually establish it in Harmony by entering key project details and uploading legacy information, applications and approvals to the system. Once a project is established, you will be able to further manage it in the system, including but not limited to the following activities:
- Amendments
- Renewals
- Reportable Events
- Project Completion
We recommend completing the setup of your existing project(s) in Harmony in advance of any required follow-on submissions (renewal, amendment).
In-flight Applications
Until December 31, 2025, continue engaging with the applicable review bodies:
-
- Post-secondary constituted Research Ethics Boards*
*For the University of Manitoba, please see the deadlines above
*Non-UM post-secondary institutions will be notified once delegation of health research reviews by their institution occurs and new applications through historical processes will stop.
-
- Provincial Health Research Privacy Committee (PHRPC)
- Relevant Health Institutional Assessments
Submit required project documentation and reports utilizing existing methods until your project is Fully Approved.
Things You Can Do Now:
- Track your project’s status (e.g., in review, partially or fully approved) and continue to follow current processes for in-flight applications.
- Submit required closure forms and documentation for projects that are completed and do not need to be transferred to Harmony.
- Watch for updates and any project-specific instructions from RITHIM, REBs, PHRPC, and health institutional partners.
- Prepare to migrate and validate your project into Harmony once fully approved.
We appreciate your cooperation and flexibility during this transition. If you have questions or concerns about your specific project or workflow, please submit a ticket at https://researchmb.freshdesk.com/support/home – we are here to help.
Thank you for your continued commitment to research excellence.
