Personal Profile Requirements
Creating Personal Profiles In Harmony
- Select “Create Project” Action
- Enter your Last name, First name, Initials in the Project Title
- Select “Personal Profile Form”
- Click “Create”
You will land on the Personal Profile Page, where you can select the “Details” Questions Page to begin.
Upon completion of all required information, the Personal Profile must be electronically signed.
- Click “Sign”
- Harmony will perform a Completeness Check to ensure all required questions have been completed
- Once the Completeness Check is passed, Sign the form by providing your Harmony Username and Password and click “Sign”
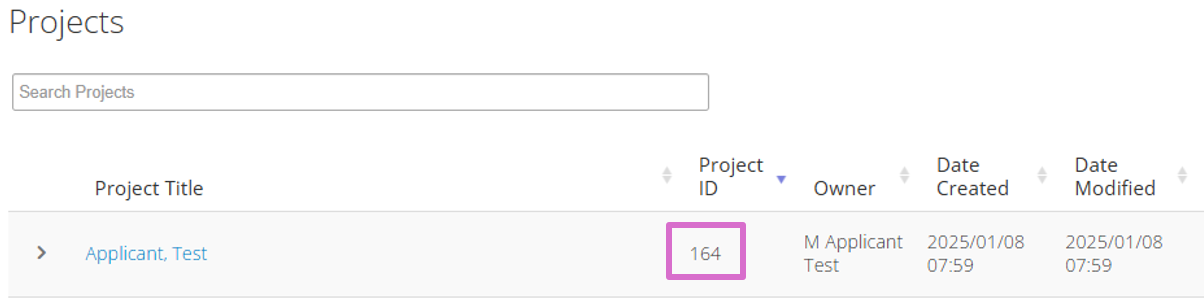
The Personal Profile Project ID (highlighted below in pink) is referenced in the RITHIM Health Research Application Form to allow Reviewers to connect to your training and qualifications. Please ensure you Personal Profile Project ID is communicated to the team member completing the RITHIM Health Research Application Form.
The following outlines the requirements for establishing a Personal Profile in Harmony:
CORE Training
The Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, 2nd edition (TCPS 2) is the official policy for human research ethics recognized by the Canadian federal granting agencies.
All individuals involved in research projects with human participants must complete the TCPS 2: Course on Research Ethics through the Government of Canada and upload their certificate of completion to the Certifications tab in their User Profile in Harmony.
PHIA Training
The Personal Health Information Act (PHIA) is designed to provide individuals with a right to examine and receive personal health information about themselves and to protect individuals from unauthorized use, disclosure or destruction of personal health information maintained by a trustee.
All personnel listed in the project Research Personnel table are required to have completed PHIA training program within the last 3 years. Your most recent completion certificate must be uploaded to the Certifications page in your Personal Profile in Harmony.
If you do not have a PHIA certificate, or it has been more than 3 years since your last certification, evidence of training will be required prior to submitting an application.
Please confirm with your home organization and the relevant data trustee(s) for your project, which PHIA training program is required. If a specific training program is not mandated by your organization, you can complete the Manitoba Health Indirect PHI Version at https://trainingtodo.com/mbhealth/secure/phia-for-individuals.asp
Curriculum Vitae
The Curriculum Vitae (CV) page of the Personal Profile must be completed for any individual who has a role of Principal Investigator, Sub-Investigator, Co-Investigator, and/or Supervisor/Advisor on a health research project.
Signature Authorities
A Signature Authority is a role within Harmony that permits the PI to designate another individual on the project team to execute activities in Harmony on their behalf. There may only be one Signature Authority, in addition to the PI, designated per project.
The PI is ultimately responsible for all aspects of their research, including the information included and submissions that are made in Harmony. All Initial Applications must be submitted by the PI. However, the PI will be able to assign the role of Signature Authority to another individual on the project team. This authority can be used for follow-on submissions when appropriate justification is provided. The PI will be copied on all submissions made by their Signature Authority. The PI may revoke or re-assign Signature Authority at any time.
Signature Authority functions can be used in the following situations:
- The PI has passed away and the Signature Authority must submit an amendment to assign a new PI; OR
- The PI is unable to submit a follow-on form during a limited period of time due to:
- Absence where access to the RITHIM-Harmony system is not possible; or
- Incapacity due to illness or injury;
- AND there is a time sensitive submission during this period requiring action from the PI or authorized delegate.
In all instances, the Signature Authority will need to provide justification in Harmony for invoking their authority. The justification will be reviewed and if it does not align with these requirements, the submission will be rejected. Submissions received from a Signature Authority will only be considered when there are well justified circumstances that prevent PIs from executing their responsibilities within Harmony.
Additional Requirement for Regulated Projects
If the project is regulated under the Health Canada Food and Drug regulations, the additional Signature Authority must be a Co-Investigator who would meet the criteria for PI for the project. For this reason, the additional Signature Authority for regulated projects is required to adhere to the same CV requirements as the PI.
Additional Requirement for Advisors/Supervisors
If the project has a Student PI, their Advisor/Supervisor must oversee and sign-off on their RITHIM Harmony submissions. For this reason, the Advisor/Supervisor for student-led projects is required to adhere to the same CV requirements as the PI.
